Description
Attention Please!
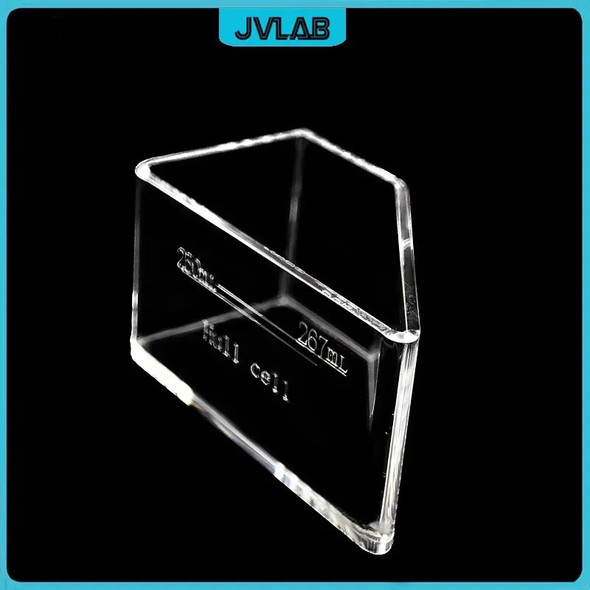
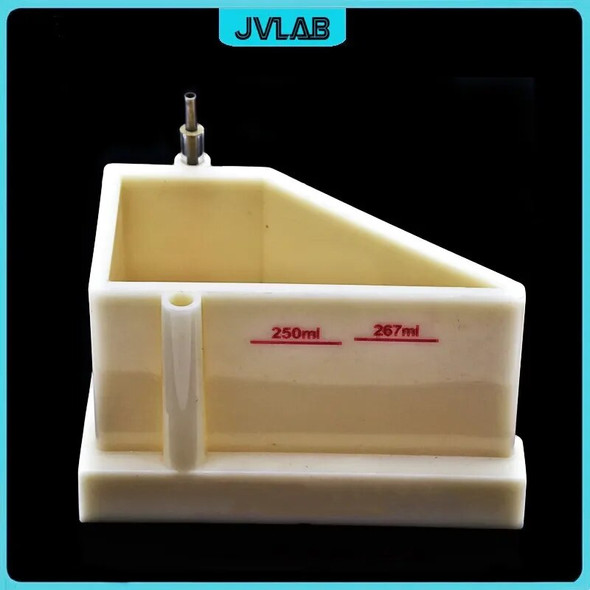
This product is only for one Electric Current Density Ruler. It does not include the brass plate and Hull Cell, if you need please click here for purchasing. Thank you.
This Hull Cell current density is used original Rohm & Haas as a reference (detailed original material please look at the last picture), material is corrosion-resistant PP colloid. In order to ensure the font and scale clear and accurate use of US imports of technology printing. Bottom of the centimeter and millimeter scale is used Japan's scale as a reference, made in accordance with the ratio of 1: 1, error within 0.2mm.
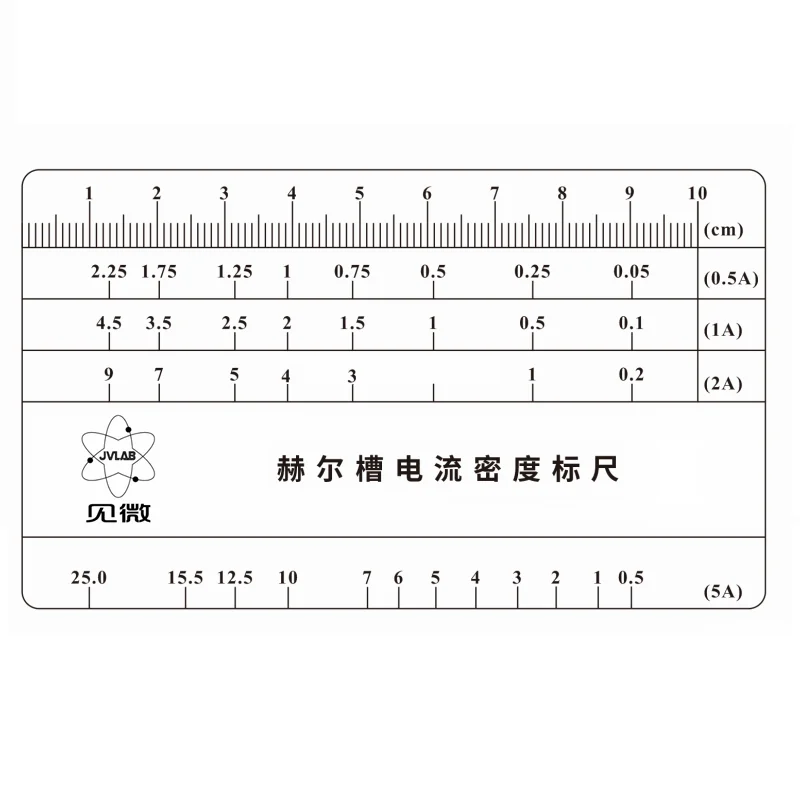
Detailed current density
Average current density (ASD) = Ampere (AMP) / Plating area (dm2)
The relationship between current density and Hull cell:
Each electroplating syrup has a certain current density operating range, such as tin alloy high-speed electroplating syrup, its operating current density range of about 2~ 30 ASD, the current density is too high coating will be rough, or even charred. Low current density coating will appear white mist or leakage coating. It is easy to visually evaluate, or to see the operating current density range of the syrup, and it can be seen that the results of the Hastelloy experiment are shown by the fact that the anode surface of the Hull Cell, Because of the cathode surface is not parallel to the plane, from the anode side of the cathode side of the current density than the anode surface away from the large, so you can compare from the high current density region to low current density area of the plating state.
So the Hull Cell experiment is all electroplating plants and potions necessary analysis tools, and its advantages as follow:
A. Chemical analysis can not find the ingredients.
B. Chemical analysis takes time-consuming ingredients.
C. Very small amount will affect the plating Ingredients.
D. The status of the bath and the future will be a bad phenomenon can know in advance.